Immune Aging Unlocked: The Key Biomarkers of Longevity
Why Your Immune System Ages You Faster And How to Stop It
This weekend I read a great article from Wu et.al. at the Buck Institute for Research on Aging. It is entitled “Immunological biomarkers of aging” and it got me thinking about all the things that we do at Concierge Medical to assess and protect your immune system.
Why? The hallmarks of aging include a robust immune system. When it goes kaput, much of our longevity is reduced. The real questions are 1) Why does it go haywire? and 2) How can we catch it early in the game to change the outcome?
Chronic Inflammation, Altered intercellular communication, Stem Cell Exhaustion, Cellular Senescence, Deregulated Nutrient sensing, Mitochondrial dysfunction all play a role here. When we think about virus or vaccine induced accelerated Immune Aging as Dr Soon Shiong has proposed, we also have to include issues of loss of proteostasis. This is especially true with Dr Bruce Patterson’s published demonstration of retained Spike Protein assays from vaccination monocyte work.
For these reasons I think it is important to write a deep dive this week into how the immune system can slowly or rapidly kill you. I will review the topics in the article in depth…. Even better, for subscribers I will delineate how to detect these issues before they end up a problem AND what to do to treat them when they are a problem.
To access the full work now. Become a paid subscriber. Your Future Self will Thank You!
For those Readers compressed for time, print out this post and read it over next weekend. Or skip to the very end for the Hi Yield review of how to manage these twin grim reapers of aging!
The Immune System’s Silent Role in Aging
Your immune system is quietly shaping how you age, working behind the scenes in ways that significantly impact your longevity. Traditionally viewed primarily as your defense against infections and illnesses, the immune system actually plays a profound and underappreciated role in the process of aging itself. Two key concepts are central to understanding this hidden connection: immunosenescence and inflammaging.
Immunosenescence refers to the gradual deterioration of your immune system as you age. Over the decades, your ability to fight infections, respond effectively to vaccines, and maintain robust immune surveillance declines significantly. This decline results from a decrease in naïve immune cells capable of recognizing new pathogens, combined with an accumulation of memory cells that often function less effectively. Coupled with this immunosenescence is inflammaging, a state of persistent, low-grade inflammation that quietly accelerates aging from within. Rather than providing protective responses, this chronic inflammation slowly erodes the integrity of tissues and organs, steadily driving the progression of age-related diseases.
Understanding immune aging is vital for longevity medicine precisely because it illuminates pathways linking your body's defensive mechanisms directly to your lifespan and healthspan. Rather than focusing solely on visible symptoms or diseases, longevity-focused medicine must address the root causes and underlying mechanisms that silently and progressively damage health. Immunosenescence and Inflammaging represent fundamental biological changes, ones intricately intertwined with chronic diseases typically associated with aging.
Surprisingly, immune aging isn't just about catching colds more easily or recovering slower from flu. It profoundly influences your risk of chronic conditions such as Alzheimer’s disease, cardiovascular disease, and even cancer. Emerging research reveals that an aged immune system not only loses its ability to clear infections effectively (or maybe these infections linger and cause problems?) but also becomes less capable of surveilling and eliminating early-stage cancer cells. Moreover, the chronic inflammation characteristic of immune aging actively promotes degenerative diseases, driving neuronal damage in Alzheimer’s and accelerating plaque formation in cardiovascular disease.
In other words, the silent decline of your immune system doesn’t just age you it actively fuels some of the most severe and difficult to treat diseases of aging. Understanding and intervening in this process is not only crucial for longevity but foundational for living healthier, longer lives.
Immunosenescence and Inflammaging: Twin Forces Accelerating Your Biological Age
Immunosenescence is the age-associated progressive deterioration of immune function, encompassing profound changes across both innate and adaptive immunity. Over time, immunosenescence undermines the immune system's fundamental roles, defending against pathogens, surveilling for malignant cells, responding effectively to vaccines, and maintaining immune homeostasis. This multifaceted decline makes elderly individuals disproportionately susceptible to infections, autoimmune conditions, cancers, and chronic inflammatory diseases, profoundly affecting overall healthspan and lifespan.
Mechanisms Driving Immunosenescence:
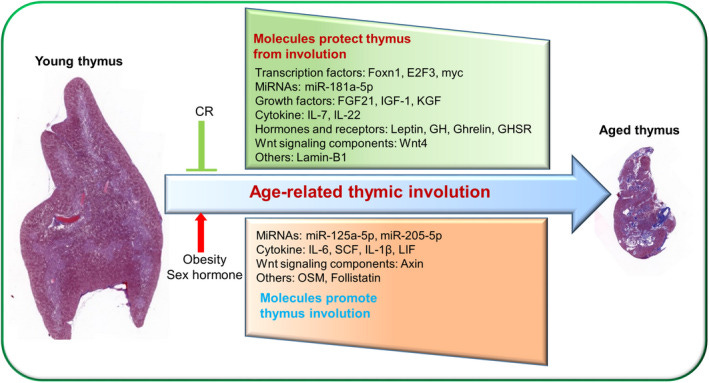
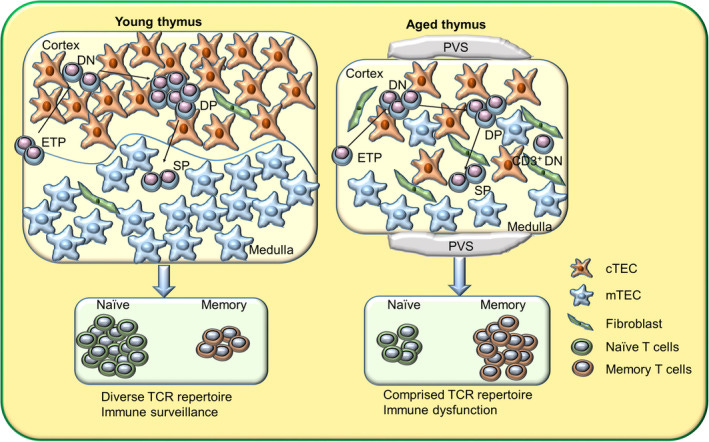
Thymic Involution and T-Cell Dysfunction:
The thymus gland, essential for producing naïve T cells during youth, gradually undergoes involution beginning as early as puberty. By mid-life, thymic output significantly diminishes, reducing the generation of new naïve T cells.
Consequently, the aged immune system relies increasingly on peripheral proliferation of existing memory T cells. Over decades, repeated antigen exposures (such as chronic viral infections like CMV or EBV) drive excessive memory T-cell expansions, dramatically restricting the diversity of the T-cell receptor (TCR) repertoire. The decreased availability of naïve T cells and the narrowed TCR repertoire severely compromises the elderly’s ability to respond to new pathogens or vaccines, exacerbating their vulnerability to novel infectious agents. Some of the work on this implicates Thymic aplasia and suggests improving its function is essential to Longevity.
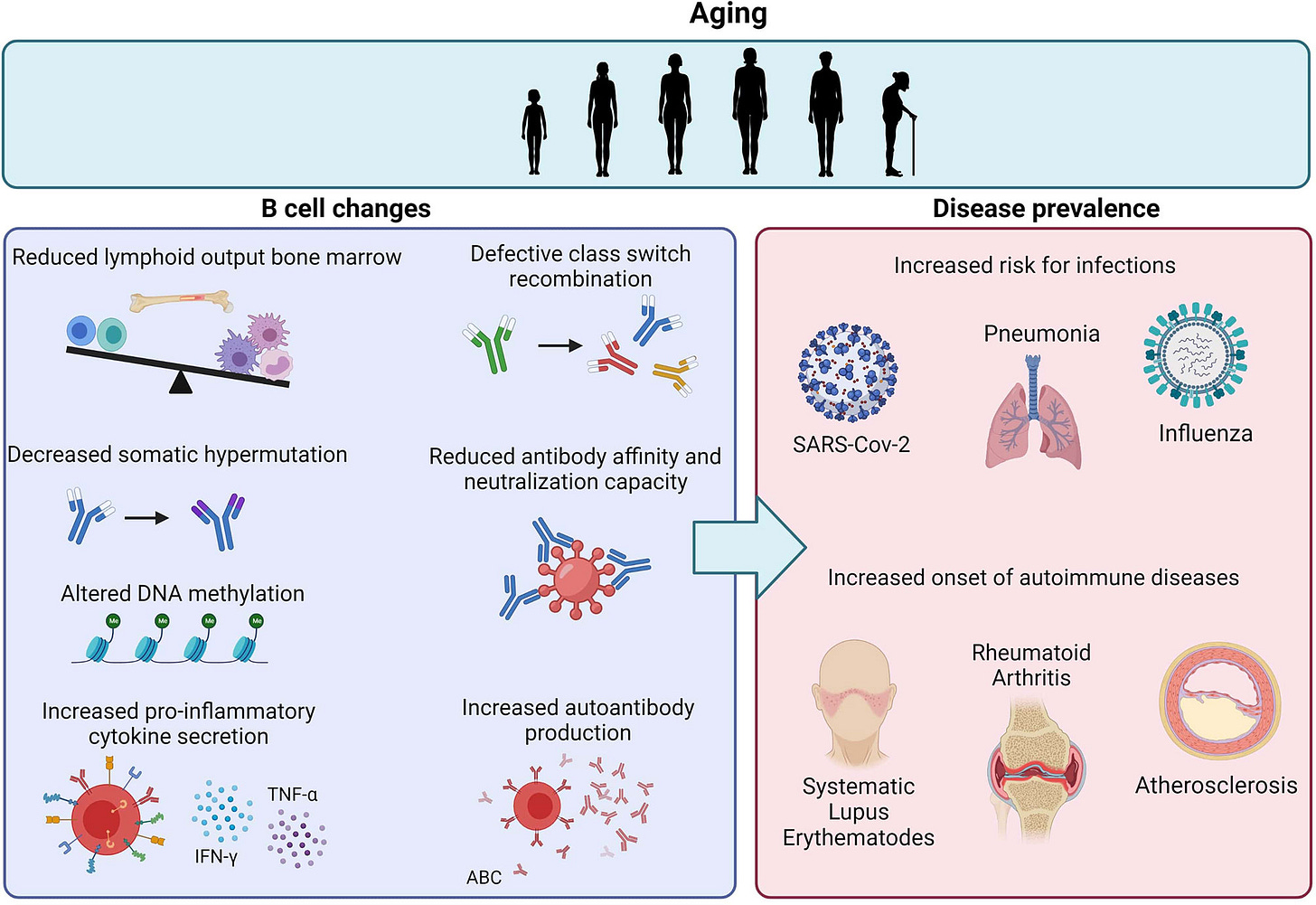
Altered B-Cell Function and Reduced Vaccine Efficacy:
With aging, significant shifts occur in B-cell biology, including reduced B-cell receptor diversity, impaired antibody class-switch recombination, and decreased somatic hypermutation. These functional changes diminish the quality, specificity, and durability of antibody responses. Clinically, this manifests as a weaker and shorter-lived protective response following vaccination or natural infection. Additionally, age-associated B cells, phenotypically distinct populations characterized by markers like CD21^low, accumulate in older individuals, contributing not only to poor vaccine responses but also heightened autoantibody production and elevated risk of autoimmune disease. Mix in the lazy/zombie NK Cells and whammo, Hashimotos.
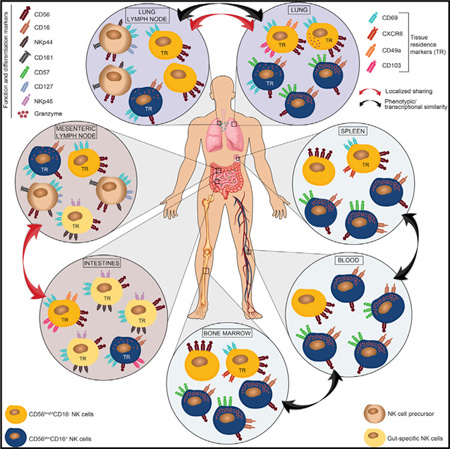
Natural Killer (NK) Cell Impairments and Reduced Cancer Surveillance:
NK cells, critical players in the innate immune system’s rapid response against virally infected and cancerous cells, become functionally compromised with age. Reduced cytotoxicity, decreased perforin and granzyme production, and altered expression of activating receptors (such as NKG2D) diminish NK cells’ effectiveness in identifying and eliminating abnormal cells. Consequently, elderly individuals experience decreased immune surveillance, significantly contributing to increased cancer incidence.
Innate Immune Cell Dysregulation and Chronic Inflammation:
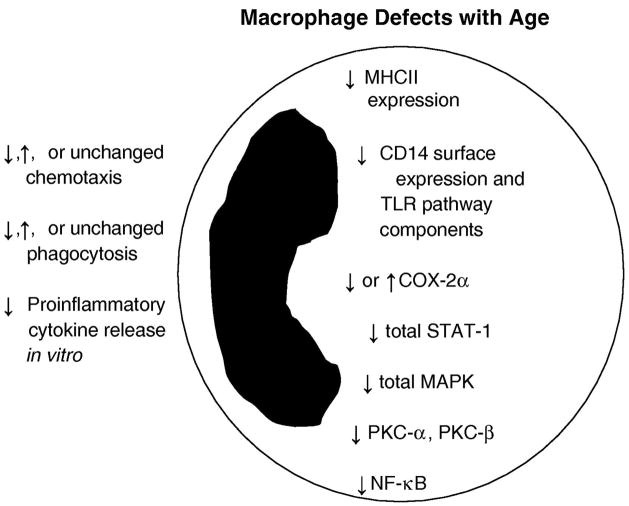
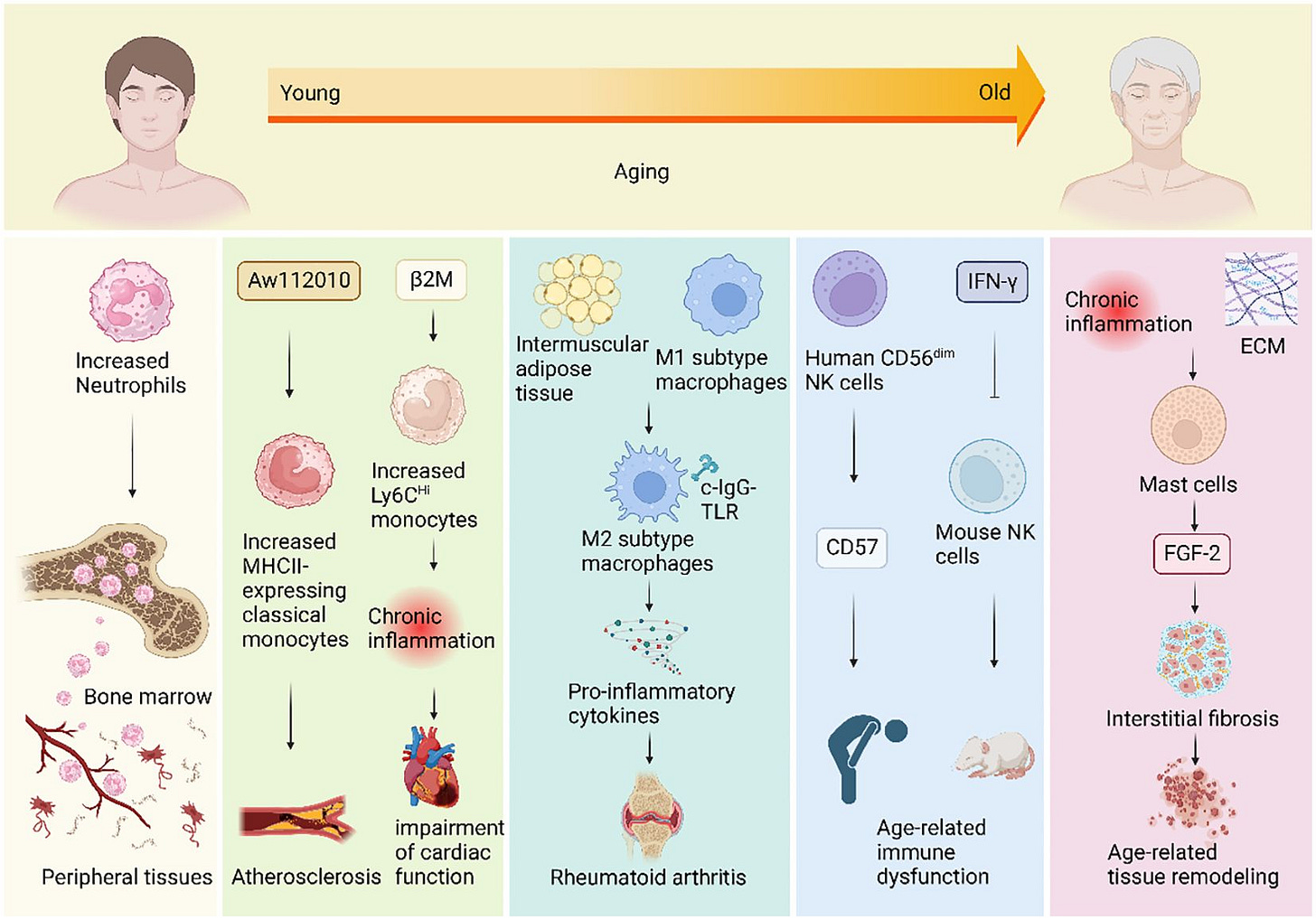
Age profoundly impacts innate immunity, altering function and phenotype of macrophages, dendritic cells, and neutrophils. With aging, macrophages demonstrate reduced phagocytic capacity and clearance of pathogens and senescent cells, thereby promoting persistent inflammation. Similarly, dendritic cells become less effective in antigen presentation, undermining adaptive immune responses. Neutrophils in older individuals exhibit impaired chemotaxis, diminished production of reactive oxygen species (ROS), and abnormal neutrophil extracellular trap (NET) formation, further weakening pathogen clearance and promoting tissue damage.
Linking Immunosenescence to the Hallmarks of Aging:
Immunosenescence interacts intimately with multiple hallmarks of aging, thereby accelerating overall biological deterioration:
Cellular Senescence:
The accumulation of senescent cells, which cease dividing yet actively secrete inflammatory mediators (senescence-associated secretory phenotype, or SASP), intensifies inflammaging. The aged immune system struggles to clear these senescent cells, perpetuating a chronic inflammatory state. Conversely, senescent immune cells, particularly exhausted T and B cells, further amplify systemic inflammation, creating a reinforcing cycle that accelerates tissue aging. Below we will highlight some of the best testing to track this.
Genomic Instability and Telomere Shortening:
Immune cells, especially proliferative T cells, are sensitive to telomere erosion due to repeated rounds of cell division. Telomere shortening drives cellular senescence and apoptosis, limiting immune cell renewal and expansion. This effect exacerbates the narrowing of immune diversity and diminishes proliferative capacity needed to mount effective responses. Many Telomere tests are done directly on Lymphocytes (WBC) but special flow cytometry can allow you to test others as well.
Mitochondrial Dysfunction:
Aging immune cells exhibit impaired mitochondrial function, reduced oxidative phosphorylation capacity, and increased ROS production. Such mitochondrial deterioration further contributes to immune cell dysfunction and chronic inflammation, notably by activating inflammatory signaling pathways like NLRP3 inflammasome. This ongoing mitochondrial stress within immune cells perpetuates systemic inflammaging.
Loss of Proteostasis and Impaired Autophagy:
The decline in autophagic flux with aging leads to compromised protein quality control within immune cells, especially in macrophages and lymphocytes. This deficiency allows damaged proteins and dysfunctional mitochondria to accumulate, exacerbating chronic inflammation and cellular dysfunction.
Epigenetic Alterations and Reduced Immune Plasticity:
Epigenetic changes, such as DNA methylation shifts and altered histone modifications, progressively restrict immune cells’ functional plasticity. These changes lock immune cells into maladaptive states (e.g., pro-inflammatory phenotypes), limiting their ability to respond effectively to new infections or environmental stimuli.
Altered Intercellular Communication:
With aging, immune signaling pathways become dysregulated. Persistent inflammation (e.g., elevated levels of IL-6, TNF-α, CXCL9) impairs tissue homeostasis and cellular communication. Chronic inflammatory signals disrupt hormonal signaling pathways and stem cell niches across multiple organ systems, driving systemic age-related deterioration.
Clinical and Therapeutic Implications for Longevity Medicine:
A comprehensive understanding of immunosenescence provides critical insights for targeted clinical interventions aiming to prolong healthspan and lifespan. Therapeutic strategies in longevity medicine increasingly prioritize restoring immune competence by:
Enhancing thymic regeneration (e.g., IL-7 therapy, thymic peptides)
Modulating chronic inflammation (e.g., mTOR inhibitors like rapamycin and everolimus, metformin, senolytics)
Improving mitochondrial health (e.g., NAD+ precursors and 1-MNA, mitochondrial-targeted antioxidants)
Restoring proteostasis and promoting autophagy (e.g., spermidine supplementation, intermittent fasting)
Lifestyle interventions (e.g., structured exercise, dietary interventions, microbiome optimization) to reduce inflammaging and enhance immune function.
Physicians focused on longevity must prioritize interventions that restore immune integrity and functionality, given the extensive evidence that immune aging substantially impacts virtually all other hallmarks of aging, significantly influencing both morbidity and mortality in the elderly population. Later on in this review we will describe strategies of detection and tools for treating Immunosenescence.
For the paid subscribers we will get very specific with dosages and lab tests.
What are you waiting for? Take the leap and become a paid subscriber. Your healthy future self will thank you!
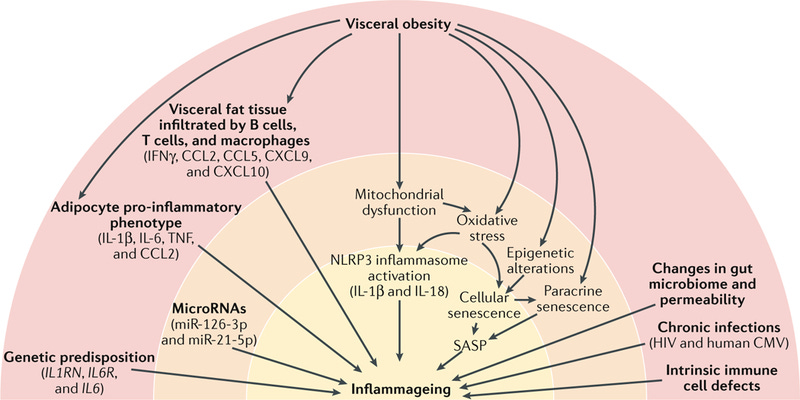
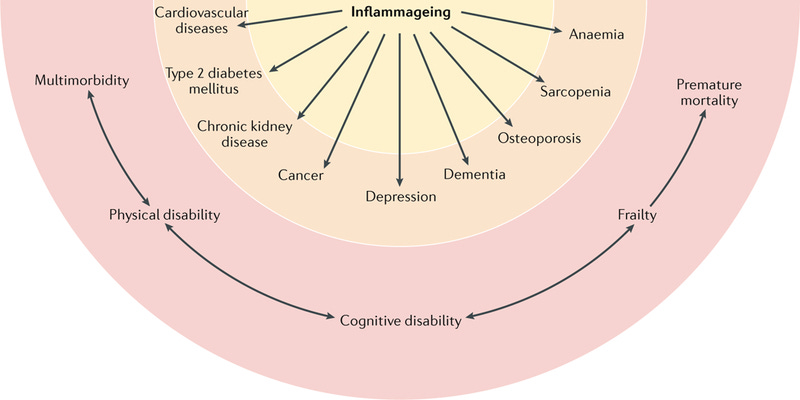
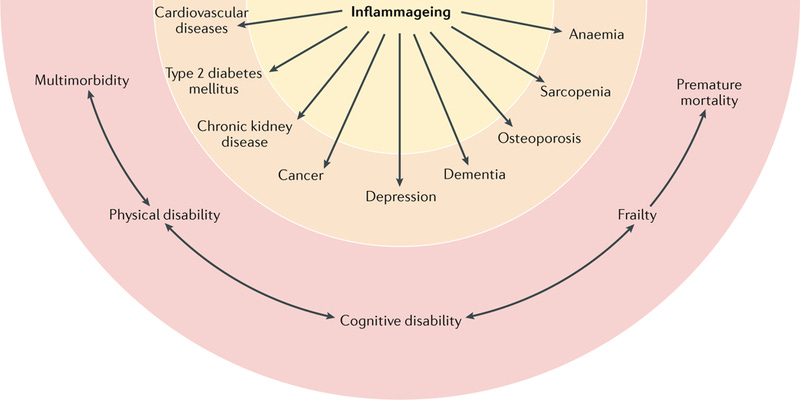
Inflammaging is defined as the persistent, low-grade inflammation that progressively develops as individuals age, even in the absence of overt infections or acute inflammatory events. Unlike acute inflammation—a protective, tightly regulated response that resolves quickly, inflammaging is chronic, systemic, and typically subclinical. This type of inflammation, while subtle and insidious, exerts profound long-term effects on organ systems, metabolic pathways, and cellular homeostasis, significantly accelerating biological aging and predisposing individuals to a multitude of age-related chronic diseases, including cardiovascular disease, diabetes, neurodegenerative diseases, autoimmune conditions, cancer, and frailty syndromes.
As physicians deeply involved in longevity and geroscience, it is crucial to appreciate that inflammaging represents a key pathological axis that intersects with and exacerbates other hallmarks of aging, serving as both a driver and consequence of the aging process.
Do you like what you are learning about? Well, don’t hide it. Share it! Your friends, family and colleagues will thank you! I will too!
One of the most useful podcasts of Thymus Function and a theory of how to Rejuvenate it!
Mechanisms Driving Inflammaging
Cellular Senescence and SASP (Senescence-Associated Secretory Phenotype):
At the cellular level, senescent cells, AKA ZOMBIE CELLS, are cells that have irreversibly ceased dividing due to DNA damage, oxidative stress, or replicative exhaustion, accumulate steadily with age. Rather than remaining inert, these cells actively secrete a complex array of pro-inflammatory cytokines, chemokines, growth factors, and proteases collectively known as the senescence-associated secretory phenotype (SASP). Key components of SASP include interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), P16INK4a, transforming growth factor-beta (TGF-β), interleukin-1 beta (IL-1β), and chemokines like CXCL9 and MCP-1. Persistent exposure to SASP factors promotes chronic systemic inflammation, disrupts tissue architecture, and damages neighboring healthy cells, effectively amplifying and perpetuating the inflammatory environment over time.
Dysfunctional Immune Cell Homeostasis:
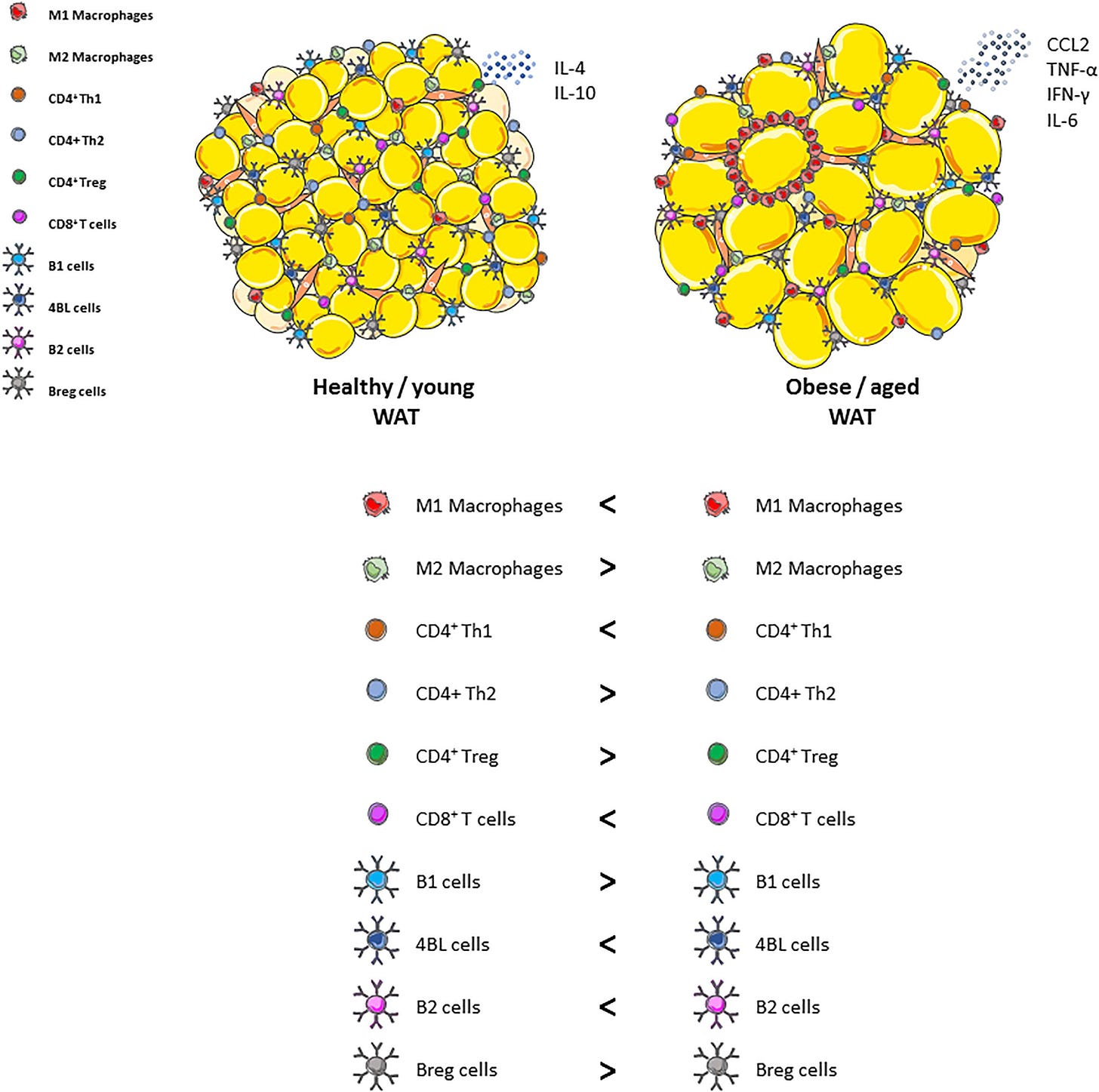
Aging fundamentally alters the immune landscape, shifting immune cell populations toward pro-inflammatory states. Aging macrophages, monocytes, dendritic cells, and neutrophils exhibit enhanced basal activation, altered cytokine profiles, and reduced regulatory mechanisms. Macrophages in aged individuals often adopt an M1-like inflammatory phenotype, characterized by excessive production of pro-inflammatory cytokines (e.g., IL-6, IL-1β, TNF-α) and reduced phagocytic capacity, impairing clearance of pathogens, senescent cells, and damaged proteins. Similarly, dendritic cells become more reactive to self-antigens, and neutrophils demonstrate defective clearance mechanisms (e.g., altered neutrophil extracellular traps or NETosis), perpetuating inflammatory cycles.
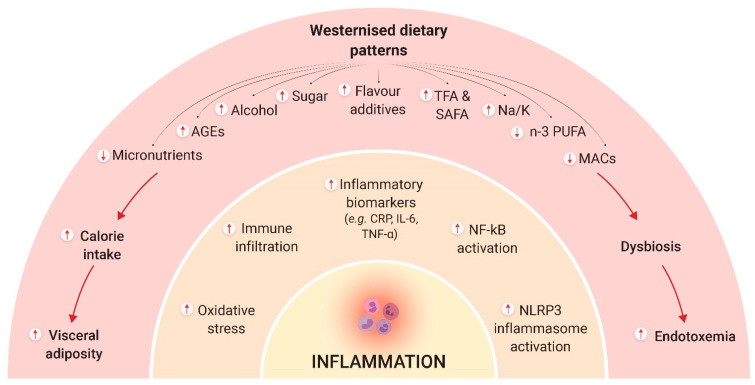
Gut Microbiota Dysbiosis and Intestinal Barrier Dysfunction:
Age-related alterations in gut microbiota composition also known as dysbiosis contribute significantly to inflammaging. Dysbiosis reduces beneficial bacterial populations while increasing pro-inflammatory bacterial species.
This imbalance compromises intestinal barrier integrity, allowing microbial metabolites, lipopolysaccharides (LPS), and other inflammatory molecules to enter systemic circulation (a phenomenon known as endotoxemia), further driving chronic inflammation. The gut microbiome thus represents a crucial target in longevity interventions aiming to mitigate inflammaging.
Metabolic Dysfunction and Adipose Tissue Inflammation:
With aging, metabolic regulation declines, characterized by insulin resistance, visceral adiposity, mitochondrial dysfunction, and dysregulated lipid metabolism. Excess visceral fat accumulates inflammatory macrophages and T cells, secreting inflammatory adipokines (leptin, resistin) and reducing anti-inflammatory adipokines (adiponectin). Mitochondrial dysfunction, prevalent in aging cells, increases reactive oxygen species (ROS), activating inflammatory pathways such as nuclear factor-kappa B (NF-κB), and NLRP3 inflammasomes. This metabolic inflammation (or "metaflammation") perpetuates chronic inflammation and further disrupts systemic metabolic homeostasis.
Impaired Autophagy and Proteostasis:
Aging significantly impairs autophagy ehich is a critical intracellular process for clearing damaged organelles, misfolded proteins, and pathogens. Reduced autophagy results in the accumulation of defective mitochondria, misfolded proteins, and aggregated molecules, directly stimulating inflammatory signaling cascades (e.g., inflammasomes activation). Consequently, diminished autophagic flux contributes profoundly to inflammaging, creating a feed-forward loop linking impaired proteostasis and chronic inflammation.
Epigenetic and Genomic Instability:
Epigenetic alterations associated with aging, including DNA methylation drift, histone modifications, and chromatin remodeling, directly influence inflammatory gene expression. Aging cells increasingly adopt epigenetic signatures favoring inflammatory pathways, heightening baseline pro-inflammatory gene transcription (e.g., IL-6, TNF-α). Moreover, age-associated genomic instability and accumulated DNA damage further stimulate inflammatory signaling via pathways like cGAS-STING, exacerbating inflammaging at the molecular level.
At Concierge Medical we do many things that others do not to assess the Immune system and it’s aging. Our favorite set of labs includes SapereX and our tools to assist with aging are legion. Want to learn how we do it? Become a patient, or easier yet, supoort out educational efforts. Become a paying subscriber to my Substack! For the cost of two starbucks a month, you can learn longevity medicine from our team in the trenches of Longevity Medicine.
Clinical and Therapeutic Implications for Longevity Medicine
Recognizing inflammaging as a central contributor to aging necessitates targeted clinical interventions to mitigate chronic inflammation and preserve healthspan. Therapeutic strategies for physicians under investigation and review include:
Senolytics and SASP Modulators: Targeting senescent cell clearance (e.g., dasatinib + quercetin, fisetin) or modulating SASP to attenuate chronic inflammation.
Metabolic Interventions: Employing metformin, caloric restriction mimetics, intermittent fasting, and dietary modulation (Mediterranean diet, ketogenic diets) to reduce metabolic inflammation. The easiest is a 72 hour fast, I’m doing one right now while typing this!
Microbiome Optimization: Prebiotic/probiotic supplementation and dietary interventions to restore microbiota balance and reduce gut-derived inflammation.
Anti-inflammatory Agents: Low-dose mTOR inhibitors (rapamycin), aspirin, omega-3 fatty acids, curcumin, resveratrol, and other polyphenols to modulate inflammatory pathways.
Lifestyle Strategies: Regular physical activity, structured exercise protocols, sleep optimization, and stress reduction techniques proven to lower chronic inflammatory biomarkers and improve overall immune and metabolic health.
Overall, the key to using these off label is to work with a physician and a process to monitor outcomes. Without measuring you are left blind guessing, whilst taking risks. Do you want to learn how to use these tools and monitor your immune system? Send me a message.
The Hidden Markers: Biomarkers of Immune Aging You Need to Know
Identifying precise and reliable biomarkers is crucial to assessing immune aging and implementing targeted interventions to promote longevity. Recent advances in immunology, powered by cutting-edge analytical tools such as machine learning and single-cell technologies, have revealed novel biomarkers that illuminate the aging process in unprecedented detail. Among the most promising developments are the Inflammatory Aging Clock (iAge), the Cell Proportion Clock, and sophisticated approaches leveraging Metabolomics to assess immune health. We are going to review the article published by Wu et.al. here.
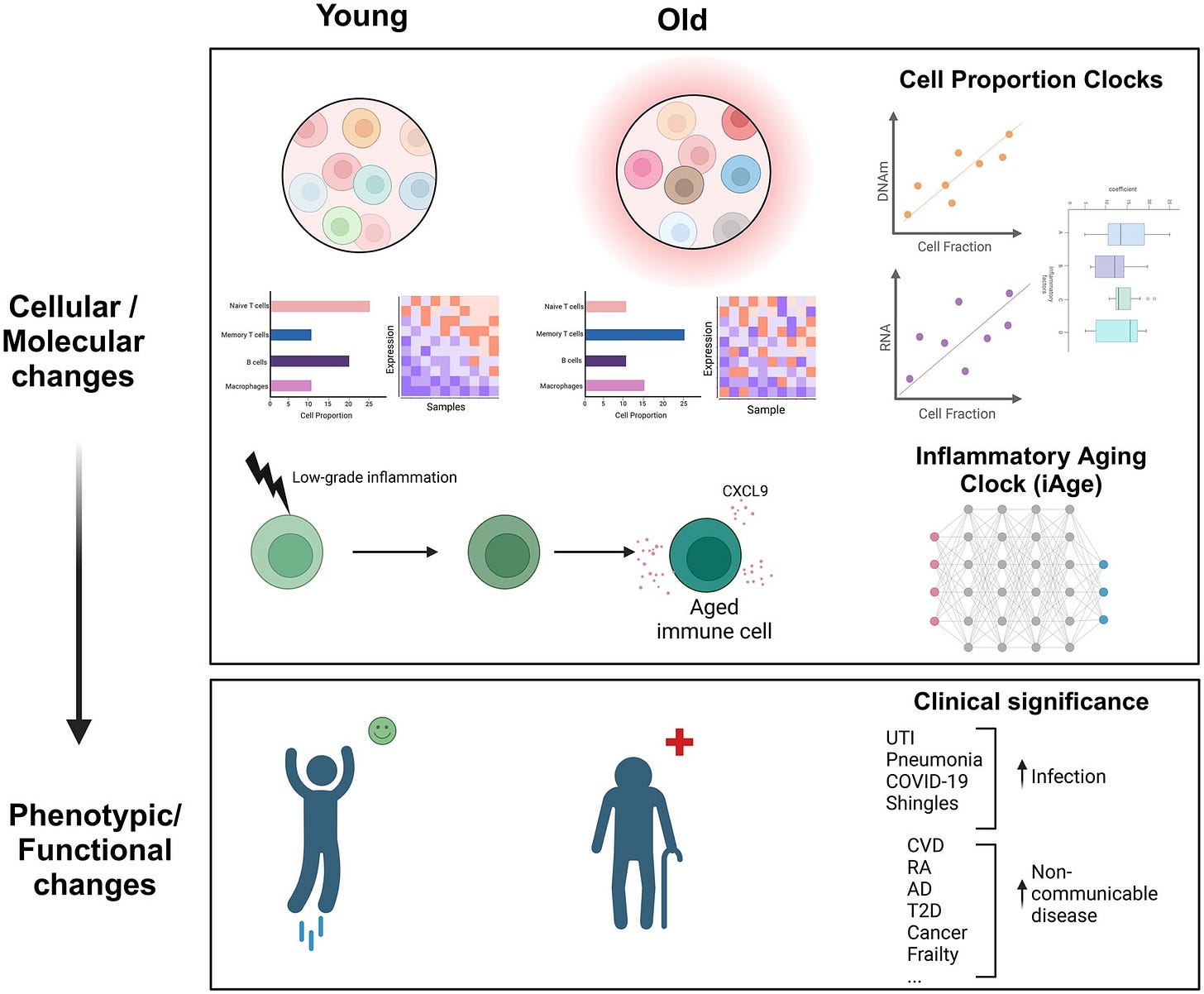
Inflammatory Aging Clock (iAge)
A significant advancement in the field of geroscience is the development of the Inflammatory Aging Clock (iAge), a biomarker platform designed using deep-learning techniques to quantify systemic inflammation and predict age-related morbidity and frailty. Through analysis of comprehensive immune datasets, researchers identified a particular cytokine, CXCL9 as a critical biomarker tightly correlated with age-related chronic diseases and functional decline. CXCL9 is involved in recruiting inflammatory cells, such as activated T cells and macrophages, and is linked to endothelial dysfunction and vascular aging. Elevated CXCL9 levels reflect chronic inflammatory signaling, vascular damage, and increased senescence burden, strongly predicting multimorbidity, cardiovascular events, and frailty syndromes in aging individuals. This inflammatory marker thus provides clinicians with actionable data, allowing for earlier identification of individuals at risk and facilitating targeted interventions to slow the aging process.
Cell Proportion Clock
Another innovative approach in quantifying biological aging involves assessing shifts in the immune cell landscape, commonly referred to as the Cell Proportion Clock. Age-related immunological shifts result in profound alterations in the composition and proportions of immune cell subsets. These include the depletion of naïve T cells, crucial for responding to novel pathogens and vaccines, alongside an increase in memory and effector T-cell populations, particularly CD8+ memory subsets. Additional characteristic shifts involve expansions in age-associated B cells (ABCs), elevated populations of senescent CD28− T cells, increased mature natural killer (NK) cell subsets (notably CD56dim CD57+ cells), and reduced diversity and proliferative capacity of lymphocytes. These proportional changes not only reflect immune aging but also actively contribute to the diminished adaptive immune response and heightened inflammatory environment characteristic of older individuals. By using flow cytometry and mass cytometry to track these immune subsets longitudinally, clinicians can now precisely monitor biological age, evaluate immune resilience, and customize immunological interventions to rejuvenate immune function.
Metabolomics: The Immune Metabolic Signature of Aging
Metabolomics, the comprehensive study of metabolites within biological systems, offers a uniquely powerful lens to observe age-related immune alterations at the molecular level. With aging, immune cells undergo significant metabolic shifts characterized by mitochondrial dysfunction, impaired oxidative phosphorylation, increased glycolytic flux, and elevated reactive oxygen species (ROS) production. This metabolic dysregulation within immune cells fuels chronic systemic inflammation, further exacerbating aging pathology. Mitochondrial impairment, particularly evident in aging macrophages and T cells, reduces energy efficiency, increases oxidative stress, and promotes the secretion of inflammatory cytokines. High-resolution metabolomic analyses detect altered energy metabolism, shifts in amino acid metabolism (notably glutamine and arginine pathways), disrupted lipid metabolism, and elevated oxidative markers indicative of systemic inflammation and aging. By characterizing this immune metabolic signature through advanced metabolomics, clinicians can identify immune metabolic dysfunction early, allowing targeted nutritional, pharmacological, or lifestyle interventions aimed at restoring metabolic homeostasis and mitigating chronic inflammation.
Visualizing Immune Aging: From Youth to Old Age
This demonstrates the transition from balanced immune homeostasis in youth, featuring abundant naïve T and B cells and robust innate responses, to an aged immune profile characterized by reduced naïve cells, expanded memory subsets, and elevated inflammatory mediators. By integrating visual and molecular biomarkers, clinicians can better grasp the complexity and progression of immune aging, enhancing their capacity to intervene effectively to promote longevity and healthspan.
Collectively, these innovative biomarkers: Inflammatory Aging Clock, Cell Proportion Clock, and Metabolomics, empower longevity-focused physicians with precise, actionable tools to evaluate immune aging and design personalized interventions. Embracing these advanced techniques and insights can significantly enhance our ability to mitigate age-related decline, improve clinical outcomes, and ultimately extend the duration and quality of human life. Our team at Concierge Medical Associates has access to cutting edge testing and study labs that help guide our treatment and reversal of immune aging.
Real-Life Impacts of an Aging Immune System
The decline of immune function with aging, immunosenescence and inflammaging, has tangible, profound consequences on real-life health outcomes, dramatically influencing disease susceptibility, progression, and clinical outcomes. Physicians involved in geroscience and longevity medicine need to thoroughly understand these clinical implications to provide effective preventive and therapeutic care.
Increased Susceptibility to Infectious Diseases
One of the most immediately apparent impacts of immune aging is a markedly heightened susceptibility to infectious diseases. Elderly individuals are significantly more vulnerable to a variety of pathogens, including viral infections such as influenza, COVID-19, respiratory syncytial virus (RSV), bacterial infections such as pneumonia and urinary tract infections (UTIs), and reactivation of latent infections like herpes zoster (shingles). The severity and mortality from COVID-19 starkly highlighted this reality, with older adults disproportionately experiencing severe complications, hospitalization, intensive care admissions, and fatalities due to immune system decline. Similarly, pneumonia remains one of the leading causes of death in elderly populations, largely due to impaired neutrophil function, reduced mucosal immunity, and diminished adaptive immune responses. Urinary tract infections, often presenting atypically in elderly patients, further underscore immune aging, characterized by decreased mucosal defenses, impaired local macrophage function, and compromised T and B-cell responses, which leads to frequent hospitalizations, cognitive decline, and increased healthcare utilization.
Reduced Vaccine Effectiveness
Do you remember yet another “failed flu shot”? Chances are, your immune system didn’t respond to it the way scientists in a lab expected it to. Another crucial consequence of immune aging is the progressive reduction in vaccine effectiveness. Older adults often exhibit impaired responses to standard vaccines such as influenza, pneumococcal, shingles, and recently, COVID-19 vaccines. This reduced efficacy stems from decreased availability of naïve T cells, limited repertoire diversity of the T-cell receptors, and diminished B-cell responses, which collectively impair the generation of robust and long-lasting antibody responses. Clinically, this means that elderly patients frequently demonstrate lower antibody titers post-vaccination, experience shorter durations of vaccine protection, and are thus at increased risk of breakthrough infections. To address these challenges, high-dose vaccines, adjuvanted formulations, and booster strategies are often required for older individuals to achieve adequate protection, underscoring the importance of tailored vaccination strategies to overcome immunosenescence. The problem with adjuvant bashing of an immune system is that it can make it worse in the end. Instead of adjuvants, we should be focusing on repairing response. We can do that. In the therapeutics section I will describe how for our subscribers.
If you haven’t started a paid subscription, why not? Drop me a line and let me know why.
Heightened Risk of Autoimmune Diseases
Immunosenescence paradoxically contributes to a higher prevalence and severity of autoimmune conditions in older adults, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS). Age-related shifts toward chronic inflammation, coupled with dysfunctional regulatory T-cells and a reduced capacity for self-tolerance, significantly enhance the risk and progression of autoimmune pathology. In rheumatoid arthritis, for example, age-associated B cells and dysfunctional CD4+ helper T cells drive chronic joint inflammation, cartilage degradation, and systemic symptoms. Lupus and MS similarly demonstrate increased disease activity and severity due to the expansion of autoreactive B-cell populations, defective regulatory T-cell function, and heightened chronic inflammatory signaling. Understanding and managing immune dysregulation is thus critical for clinicians treating autoimmune diseases in older patients, necessitating therapeutic interventions that restore immune balance and control inflammaging.
Cancer Risk and Impaired Immune Surveillance
An aging immune system also profoundly affects cancer incidence, progression, and treatment responses. The impaired function of natural killer (NK) cells and cytotoxic T cells, a hallmark of immunosenescence, significantly weakens immune surveillance against cancer cells, increasing the risk of tumor initiation, growth, and metastasis. Specifically, age-associated dysfunction in NK cells reduces their cytotoxic capabilities, resulting in less effective recognition and elimination of emerging tumor cells.
Moreover, aged cytotoxic T cells exhibit diminished proliferative capacity, decreased production of perforin and granzyme, and increased expression of exhaustion markers such as PD-1, collectively diminishing antitumor responses. Simultaneously, chronic inflammation (inflammaging) creates a tumor-permissive environment, promoting mutations, angiogenesis, tissue remodeling, and metastasis.
Clinically, these combined immune impairments result in significantly higher incidences of multiple malignancies, such as lung, breast, prostate, and colorectal cancers, in older populations. Therefore, enhancing immune surveillance through targeted immunotherapies, senolytics, and strategies to reduce chronic inflammation becomes increasingly critical to reducing cancer burden and improving treatment outcomes in aging patients.
Together, these real-life impacts, heightened infection susceptibility, diminished vaccine responsiveness, elevated autoimmune risks, and increased cancer prevalence, highlight the urgent clinical need to address immune aging comprehensively. Targeted therapeutic strategies, tailored preventive measures, and personalized medical approaches must be prioritized by physicians to enhance immune health, extend healthspan, and significantly improve quality of life for aging populations.
Why Does Your Immune System Age?
Understanding the fundamental drivers behind immune aging is essential for physicians seeking to extend healthspan and longevity. Immune aging does not occur randomly; rather, it is driven by several interconnected cellular, molecular, and environmental factors that progressively undermine immune function. Key primary drivers include mitochondrial dysfunction, telomere shortening and DNA damage, impaired proteostasis and autophagy, and cumulative environmental exposures collectively termed the EXPOSOME.
Mitochondrial Dysfunction: Increased Oxidative Stress and Pro-inflammatory Metabolism
Mitochondria are central to maintaining immune cell functionality, providing the energy required for immune responses through oxidative phosphorylation. With advancing age, however, mitochondrial integrity deteriorates significantly, manifesting as reduced bioenergetic capacity, impaired oxidative phosphorylation, increased production of reactive oxygen species (ROS), and heightened oxidative stress. This mitochondrial dysfunction is particularly pronounced in aging immune cells, such as macrophages and T cells, which depend heavily on mitochondrial metabolism. Increased ROS levels directly damage cellular components, including lipids, proteins, and DNA, further exacerbating immune dysfunction. Simultaneously, damaged mitochondria promote pro-inflammatory signaling cascades, activating inflammatory complexes like the NLRP3 inflammasome, thus driving chronic, low-grade systemic inflammation known as inflammaging. Clinically, mitochondrial impairment in immune cells compromises pathogen clearance, diminishes vaccine responsiveness, and facilitates progression of age-related inflammatory diseases, highlighting mitochondria-targeted interventions (e.g., NAD+ precursors, mitochondrial antioxidants, and metabolic therapies) as promising therapeutic avenues for reducing immune aging.
Telomere Shortening and DNA Damage: Accelerating Immune Cell Senescence
Telomeres, repetitive DNA sequences protecting chromosome ends, progressively shorten with each cell division, acting as a molecular clock reflecting cellular aging. In immune cells, particularly proliferative lymphocytes like T cells, telomere shortening occurs rapidly due to repeated cycles of antigen-driven expansion. Critically shortened telomeres trigger DNA damage responses, driving immune cells into senescence, a state of permanent growth arrest accompanied by a chronic secretion of inflammatory mediators (the SASP phenotype). Concurrently, accumulated DNA damage, exacerbated by chronic oxidative stress and impaired DNA repair mechanisms, further accelerates immune cell dysfunction and senescence. Clinically, this manifests as reduced immune repertoire diversity, impaired regenerative capacity, weakened pathogen responses, and increased susceptibility to malignancies. Understanding and addressing telomere biology through therapies aimed at stabilizing telomere length or mitigating DNA damage (such as senolytics, telomerase activation strategies, and antioxidants) become crucial in longevity medicine to preserve robust immune functionality.
Proteostasis Impairment and Autophagy: Failure to Clear Damaged Cellular Components
Effective immune cell function critically depends upon maintaining cellular proteostasis, the homeostasis of proteins ensuring proper folding, repair, and degradation of damaged proteins. With age, the intricate cellular machinery responsible for protein homeostasis (proteostasis) progressively deteriorates, compromising mechanisms such as the ubiquitin-proteasome system and autophagy. Autophagy, a tightly regulated intracellular degradation pathway crucial for clearing damaged organelles, misfolded proteins, and intracellular pathogens, is significantly impaired in aging immune cells. Reduced autophagic flux leads to the accumulation of dysfunctional mitochondria, aggregated proteins, and inflammatory intracellular debris. This impairment triggers innate inflammatory pathways, such as inflammasomes and NF-κB signaling, driving chronic inflammation and immune cell dysfunction. Clinically, compromised autophagy and proteostasis contribute significantly to age-related immune deterioration, manifested by impaired pathogen clearance, heightened inflammatory states, and exacerbation of autoimmune diseases. Therapeutically enhancing proteostasis and autophagy through caloric restriction, intermittent fasting, pharmacological agents like rapamycin, spermidine, and lifestyle interventions—emerges as a powerful strategy to restore immune resilience and mitigate immune aging.
Environmental Exposome: Chronic Infections, Lifestyle, Diet, and Stress
The cumulative impact of lifelong environmental exposures, termed the exposome, profoundly accelerates immune aging. Chronic infections, such as persistent cytomegalovirus (CMV) or Epstein-Barr virus (EBV) infections, drive continuous low-level immune activation, exhausting immune cell reserves and narrowing the immune repertoire over time. Additionally, sedentary lifestyles characterized by minimal physical activity exacerbate systemic inflammation and weaken immune responses, whereas regular physical activity robustly improves immune cell functionality and reduces chronic inflammatory states. Diet significantly influences immune aging: diets rich in refined sugars, processed foods, and pro-inflammatory fats contribute to gut microbiome dysbiosis, compromised intestinal barriers, endotoxemia, and chronic systemic inflammation, whereas diets emphasizing whole foods, fiber, antioxidants, and healthy fats mitigate these inflammatory cascades. Psychological and physiological stressors also accelerate immune aging, activating stress-response pathways (e.g., cortisol dysregulation, sympathetic nervous system hyperactivity) that impair immune regulation and enhance inflammatory signaling. Physicians addressing longevity must prioritize comprehensive strategies….incorporating infection management, regular physical activity, nutritional optimization, microbiome restoration, and stress reduction….to mitigate the harmful impact of environmental exposures and significantly slow immune aging.
Emerging evidence strongly suggests that chronic exposure to environmental toxins significantly accelerates immunosenescence and inflammaging, profoundly influencing immune function and overall longevity. Environmental toxins, including heavy metals, air pollutants, pesticides, endocrine-disrupting chemicals, and microplastics, pose a continuous, often invisible threat, quietly contributing to chronic inflammation, oxidative stress, immune dysregulation, and accelerated biological aging.
Heavy Metals and Immune Dysfunction
Heavy metals such as lead, mercury, cadmium, and arsenic, frequently encountered through contaminated water, air pollution, seafood, and industrial exposure, significantly impair immune function. Chronic exposure to these metals induces persistent oxidative stress, mitochondrial dysfunction, and inflammation, ultimately triggering immune cell senescence and impaired adaptive responses. Mercury exposure, for instance, has been linked to reduced T-cell function, impaired cytokine signaling, and elevated inflammatory markers (Gardner & Nyland, 2016). Cadmium, found in tobacco smoke and industrial pollutants, strongly induces chronic inflammation, weakens innate immune defenses, and promotes T-cell exhaustion, dramatically accelerating immune aging (Fagerberg et al., 2015). Further studies like the one below have show that vial persistence and reactivation are associated with higher levels of heavy metals.
Air Pollution and Chronic Immune Activation
Ambient air pollutants especially fine particulate matter (PM2.5), nitrogen dioxide (NO₂), ozone (O₃), and polycyclic aromatic hydrocarbons (PAHs) have been consistently linked to chronic inflammation and immune system decline. Exposure to PM2.5 and ozone, prevalent in urban environments, leads to persistent activation of inflammatory signaling pathways such as NF-κB and NLRP3 inflammasomes, directly promoting inflammaging and exacerbating chronic respiratory diseases, cardiovascular disease, and metabolic dysfunction (Pope et al., 2016; Brook et al., 2017). Long-term exposure to air pollution has also been associated with impaired immune surveillance, reduced vaccine responses, increased susceptibility to respiratory infections, and accelerated progression of chronic diseases (Yang & Omaye, 2009).
Learn about the rest of the exposome and how to protect your immune system from accelerated aging. Your future self will thank you!
Keep reading with a 7-day free trial
Subscribe to Longevity Insider with Dr. Murphy to keep reading this post and get 7 days of free access to the full post archives.