Transforming Medicine: Microtubules in Longevity, From Molecular Mechanics to Quantum Consciousness
Missing Secrets of Longevity Solved
Why Longevity Physicians and Patients Should Deeply Understand Microtubules
Microtubules are one of biology’s most fascinating molecular structures, pivotal to processes from cellular division to brain function. Although initially discovered as structural scaffolds, they have been increasingly appreciated as active regulators of aging, neurodegenerative diseases, epigenetic states, and even cognitive phenomena such as consciousness.
In this lecture-style post, we will journey deeply into:
Molecular Biology of Microtubules
Microtubules in Aging and Neurodegeneration
Quantum Biology of Microtubules
Clinical and Therapeutic Implications
1. Microtubule Structure: Precise Molecular Architecture
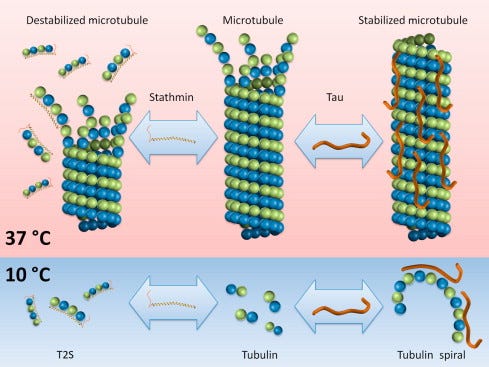
Microtubules are sophisticated, highly conserved cylindrical polymers, measuring approximately 25 nanometers in diameter, that are present in virtually all eukaryotic cells. Their uniform and precise structural organization enables their pivotal roles in maintaining cellular integrity, mediating intracellular transport, and orchestrating cell division. At their most fundamental level, microtubules consist of linear structural units termed protofilaments. A protofilament is a polymeric chain built from repeating heterodimers composed of α-tubulin and β-tubulin proteins. Exactly thirteen protofilaments associate laterally, aligning parallel to each other, forming the cylindrical lattice characteristic of microtubules.
Each α/β-tubulin dimer represents the basic building block of microtubules. Structurally, the α- and β-tubulin subunits are each approximately 55 kDa in size, and each monomer binds one molecule of Guanosine Triphosphate (GTP). The α-tubulin binds its GTP molecule non-exchangeably, meaning the GTP remains intact and is never hydrolyzed. In contrast, β-tubulin binds its GTP in an exchangeable manner, meaning that this bound GTP is destined for hydrolysis to GDP (Guanosine Diphosphate). This hydrolysis reaction is not merely an incidental event; rather, it provides critical energy and structural signals that dictate the stability and dynamics of the microtubule.
The formation of the α/β-tubulin heterodimer itself is a carefully regulated, energy-dependent process. When β-tubulin binds GTP, it assumes a stable, energetically favorable conformation called the "GTP-bound state." GTP-bound tubulin dimers are highly favored for addition to the growing end (known as the plus-end) of the microtubule. However, soon after their incorporation into the polymer lattice, the GTP bound to β-tubulin is hydrolyzed to GDP. This GTP-to-GDP hydrolysis event occurs at the interface between adjacent subunits within the protofilament lattice. Critically, the hydrolysis of GTP causes a significant conformational shift within the β-tubulin subunit, weakening its interactions with neighboring dimers and reducing overall microtubule stability, setting the stage for microtubule dynamics.
2. Dynamic Instability: An Essential Cellular Phenomenon
Microtubules are remarkable for their unique property termed "dynamic instability," first described by Tim Mitchison and Marc Kirschner in the early 1980s. Dynamic instability is characterized by rapid, spontaneous transitions between phases of growth (polymerization) and shrinkage (depolymerization). This alternating pattern allows cells to dynamically reorganize their internal architecture, making microtubules highly responsive cellular structures essential for adaptability.
During the polymerization or "rescue" phase, microtubules rapidly elongate by adding GTP-bound α/β-tubulin dimers onto their plus-end. This continuous addition forms a stabilizing region at the tip known as a "GTP cap," composed of recently added dimers whose GTP has not yet been hydrolyzed. The presence of this GTP cap maintains protofilament cohesion, thereby promoting continued growth.
In contrast, during the depolymerization or "catastrophe" phase, the GTP bound to β-tubulin within the lattice hydrolyzes to GDP. GDP-bound protofilaments are structurally less stable, and this instability rapidly propagates, resulting in a peeling away of protofilaments from the microtubule tip. This rapid structural disintegration occurs over mere seconds, disassembling microtubules and enabling quick rearrangement of cellular architecture.
Dynamic instability is essential in various cellular processes critical for organismal health and longevity. For instance, it allows precise control of chromosome segregation during cell division via mitotic spindles. It is also pivotal in facilitating rapid reconfiguration of intracellular transport pathways, essential for transporting organelles, vesicles, and signaling molecules. Furthermore, dynamic microtubule turnover underpins cellular migration, wound healing, and immune cell chemotaxis—processes vital for tissue homeostasis and longevity.
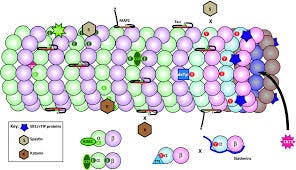
3. Microtubule-Associated Proteins (MAPs): Regulatory Partners
Microtubules do not function alone. Their stability, dynamics, and functional specificity depend critically upon interactions with a diverse group of proteins collectively termed Microtubule-Associated Proteins (MAPs). These proteins profoundly modulate microtubule behavior and cellular outcomes.
Structural MAPs physically associate with the surface of microtubules, influencing their stability, spacing, and bundling. A classic example is the tau protein, primarily expressed in neurons, which enhances microtubule stability by cross-linking adjacent protofilaments. However, pathological hyperphosphorylation of tau disrupts its binding to microtubules, leading to neurofibrillary tangles—hallmarks of Alzheimer's disease and related neurodegenerative conditions. Other structural MAPs such as MAP2 and MAP1B are crucial for maintaining dendritic architecture and synaptic plasticity, playing vital roles in neuronal function and longevity.
Motor proteins represent another critical class of MAPs, functioning as intracellular cargo transporters that traverse microtubule tracks using ATP as fuel. Kinesins are motor proteins that predominantly move cargoes toward microtubule plus-ends (anterograde transport). They are vital for delivering synaptic vesicles, mitochondria, and signaling molecules, and they play essential roles in neuronal growth and synaptic plasticity. Dyneins, conversely, transport cargo toward microtubule minus-ends (retrograde transport). They are critically involved in recycling neurotransmitters, transporting damaged organelles for degradation via autophagy, and regulating immune responses.
Additional regulatory MAPs, including EB proteins, CLASP proteins, and stathmin, transiently associate with microtubule ends and directly modulate their dynamic instability. EB proteins preferentially bind growing microtubule ends, facilitating recruitment of other regulatory molecules and finely tuning polymerization rates. CLASP proteins help stabilize microtubules during moments of depolymerization, effectively promoting microtubule longevity ("catastrophe rescue"). Such precise regulation of microtubule dynamics is critical for maintaining cellular structure, directional transport, and overall cellular health.
4. Clinical and Biological Relevance for Longevity Medicine
The precise structural organization and dynamic regulation of microtubules significantly influence cellular health and longevity. Proper microtubule function is indispensable for neuronal health; disruptions in microtubule stability and transport are closely linked to neurodegenerative diseases such as Alzheimer's, Parkinson's, and ALS. These conditions underscore the therapeutic potential of targeting microtubules in longevity medicine.
Moreover, microtubule dysfunction contributes directly to cellular aging, or senescence, by impairing critical processes such as autophagy, mitochondrial transport, genomic stability, and inflammation control ("inflammaging"). Targeting microtubules therapeutically with stabilizing agents—such as epothilones, noscapine derivatives, and HDAC6 inhibitors—holds promise for slowing neurodegeneration, enhancing neuronal resilience, and extending cellular healthspan. Such therapeutic strategies align directly with the overarching goals of longevity medicine, emphasizing microtubules as not merely structural components, but as central elements in the pursuit of extended health and lifespan.
Microtubules in Aging and Neurodegenerative Disease
Why Microtubules Matter in Aging
Microtubules are not static structural elements; they are dynamic molecular machines whose integrity critically impacts cellular health. As cells age, microtubules experience significant structural and functional decline, becoming increasingly unstable and susceptible to disruption. This deterioration contributes profoundly to the aging phenotype observed at the cellular, tissue, and organismal levels. Understanding these processes is crucial for clinicians interested in longevity, as microtubule dysfunction directly intersects with several hallmark features of cellular senescence and age-related diseases.
Mechanisms of Microtubule Decline in Aging
One of the central features of aging cells is the progressive impairment of microtubule structure and dynamics. With advancing age, microtubules become less efficient at maintaining their dynamic instability, leading to decreased ability to respond to cellular signals and compromised cellular functionality. Several cellular consequences arise directly from these microtubule disturbances:
1. Impaired Cell Division and Genomic Instability
During healthy cell division, microtubules orchestrate the accurate segregation of chromosomes by forming the mitotic spindle. However, as cells age, spindle formation and function become impaired. Microtubules exhibit decreased polymerization efficiency, reduced stability, and increased frequency of depolymerization events ("catastrophes"), resulting in abnormal spindle configurations. These aberrations significantly raise the risk of chromosomal mis-segregation and aneuploidy (abnormal chromosome number), contributing to genomic instability—a major driver of cellular senescence and aging-related pathologies including cancer.
2. Defective Mitochondrial Transport and Oxidative Stress
Healthy cellular function depends heavily on the regulated distribution of mitochondria, which rely on microtubules for transport throughout the cell. Neurons, for example, require robust mitochondrial transport to supply energy to synapses and to remove damaged mitochondria from distal regions of axons. With age, microtubules become structurally weakened and their interactions with motor proteins (kinesins and dyneins) become less efficient. Consequently, mitochondrial distribution becomes impaired, with mitochondria accumulating aberrantly, failing to deliver ATP effectively, and suffering increased oxidative damage due to reduced mitophagy (removal of defective mitochondria). This accumulation of damaged mitochondria exacerbates oxidative stress, further harming cellular structures, accelerating senescence, and contributing directly to age-related degeneration.
3. Dysregulated Autophagy and Impaired Proteostasis
Microtubules form essential intracellular highways guiding autophagosomes (organelles responsible for sequestering cellular waste) toward lysosomes, where degradation of cellular debris and damaged proteins occurs. Aging-associated instability of microtubules significantly disrupts this intracellular trafficking, impairing autophagy and proteostasis—the cell’s capability to maintain protein homeostasis. In turn, defective autophagy leads to accumulation of misfolded proteins, further stressing the cell, exacerbating inflammation ("inflammaging"), and accelerating cellular aging.
Alzheimer's Disease and Tauopathies: A Microtubule-Centric Case Study
Microtubule dysfunction is exemplified in Alzheimer’s disease (AD) and related tauopathies—disorders defined by the pathological aggregation of tau protein. Tau, in its healthy state, stabilizes neuronal microtubules. In tauopathies, including AD, tau becomes hyperphosphorylated, resulting in its pathological dissociation from microtubules, thereby destabilizing them.
Step-by-step Pathogenesis in Alzheimer's Disease:
Step 1: Tau Hyperphosphorylation
Under physiological conditions, tau protein tightly binds and stabilizes neuronal microtubules. However, in Alzheimer's, aberrant activation of kinases, notably glycogen synthase kinase-3 beta (GSK-3β) and cyclin-dependent kinase 5 (CDK5), leads to excessive phosphorylation of tau. Hyperphosphorylation alters tau’s structural conformation, reducing its affinity for microtubules.
Step 2: Tau Dissociation from Microtubules
The loss of tau binding destabilizes neuronal microtubules significantly. Without the supportive scaffolding provided by tau, microtubules begin to depolymerize frequently, resulting in impaired structural integrity and decreased dynamic stability.
Step 3: Formation of Neurofibrillary Tangles (NFTs)
Free hyperphosphorylated tau proteins, now dissociated from microtubules, aggregate intracellularly to form insoluble, filamentous structures termed Neurofibrillary Tangles (NFTs). NFTs are toxic aggregates that accumulate progressively, disrupting cellular functions.
Step 4: Disrupted Axonal Transport and Synaptic Dysfunction
Microtubule destabilization severely compromises axonal transport, critical for delivering mitochondria, synaptic vesicles, neurotrophins, and other vital molecules along neuronal axons. The impaired delivery of these essential components results in progressive synaptic dysfunction, characterized by synapse loss, impaired neurotransmission, and cognitive deficits.
Step 5: Neuronal Death and Cognitive Decline
The cumulative result of chronic microtubule destabilization and NFT accumulation is neuronal degeneration and death. The widespread neuronal loss underlies the progressive cognitive impairment and clinical dementia observed in Alzheimer's disease.
Clinical Relevance: Microtubule-Stabilizing Drugs and Longevity Medicine
Given the centrality of microtubules in neurodegeneration, targeted pharmacological stabilization of microtubules has emerged as a promising therapeutic strategy. Microtubule-stabilizing drugs are currently under rigorous investigation for their potential to halt or reverse neuronal damage in Alzheimer’s disease and tauopathies:
Epothilones:
Epothilones, originally identified as anticancer agents, effectively stabilize microtubules even in the presence of pathological tau. Preclinical models demonstrate that epothilones restore axonal transport, improve neuronal viability, and reverse cognitive impairment by stabilizing neuronal microtubules. Ongoing clinical trials aim to translate these promising findings into effective Alzheimer’s therapies.
Noscapine Analogs:
Noscapine, an opium-derived compound, and its analogs, represent another promising class of microtubule-stabilizing agents. Noscapine derivatives stabilize microtubules without causing overt toxicity, and preclinical studies show their ability to reduce tau aggregation and improve neuronal health significantly.
HDAC6 Inhibitors:
Histone deacetylase-6 (HDAC6) modulates microtubule dynamics via deacetylation of tubulin. Inhibitors of HDAC6 enhance microtubule acetylation, promoting increased stability. These drugs improve mitochondrial transport, enhance autophagy, and significantly reduce tau-induced neurotoxicity in experimental models.
Microtubules as Longevity Targets
The deterioration of microtubule structure and function is not merely a byproduct of aging—it actively accelerates senescence and neuronal death. Alzheimer’s disease provides a stark example of how microtubule dysfunction can catalyze widespread neurodegeneration and cognitive decline. However, therapeutic stabilization of microtubules offers significant promise. For clinicians and researchers in longevity medicine, microtubules represent an essential therapeutic target. Enhancing microtubule stability has the potential not only to mitigate symptoms but fundamentally to improve cellular health, extend neuronal function, and enhance lifespan and healthspan, positioning microtubules as a pivotal focus in the quest to understand and combat aging.
Quantum Biology—A Final Frontier?
Quantum Biology and the Challenge to Classical Thinking
Quantum biology is a burgeoning field investigating the previously unthinkable: quantum mechanical phenomena at work in biological systems. Traditional biological sciences have long operated under the assumption that quantum effects—strange behaviors typically observed at extremely low temperatures and microscopic scales—could not significantly influence biological processes at the warm, macroscopic conditions within living organisms. Yet, growing experimental evidence and theoretical advances suggest quantum mechanics might indeed play a pivotal role in essential biological processes, including enzyme catalysis, photosynthesis, olfaction, and, most intriguingly, neuronal function and consciousness itself.
To grasp the revolutionary nature of these ideas, longevity physicians must first build an intuition for fundamental quantum mechanical concepts and their possible relevance to cellular biology—particularly within the context of neuronal microtubules.
The Orch OR Theory of Consciousness: A Revolutionary Proposal
Historical Context and the Emergence of Orch OR
In the early 1990s, the landscape of cognitive neuroscience and theoretical physics was profoundly altered by a provocative hypothesis proposed by Sir Roger Penrose, a mathematician and Nobel laureate in Physics, and Dr. Stuart Hameroff, an anesthesiologist with extensive expertise in cellular biology. Their theory, named Orchestrated Objective Reduction (Orch OR), posited a groundbreaking idea: that consciousness arises not merely from classical neuronal firing patterns but from intricate quantum computations occurring within neuronal microtubules. Penrose, inspired by fundamental issues in physics and mathematics regarding the nature of consciousness, joined forces with Hameroff, who had long been intrigued by the mechanisms of anesthetic action on neurons. Together, they proposed that microtubules, previously viewed primarily as structural scaffolds, could facilitate quantum-level processes, potentially linking quantum mechanics directly to the emergence of consciousness.
An excellent 6 Minute primer, worthy of your time:
Essential Quantum Mechanics for Longevity Physicians
To fully appreciate Orch OR, physicians must first master several foundational quantum mechanical concepts that underpin the theory.
Quantum Coherence
In quantum mechanics, coherence refers to the remarkable ability of quantum systems to exist simultaneously in multiple states—a phenomenon known as "superposition." Imagine a system not being confined to one single state but simultaneously occupying multiple possibilities. Quantum coherence allows these possibilities to interact constructively or destructively, producing interference patterns analogous to ripples intersecting on a pond’s surface.
Quantum Entanglement
Entanglement describes quantum states of two or more particles becoming interconnected in such a profound manner that the state of one instantaneously influences the state of the other, regardless of physical separation—a phenomenon Einstein famously termed "spooky action at a distance." Entangled particles behave as a single, indivisible system, an effect increasingly recognized for its potential utility in ultra-fast and powerful computations.
If you have the time, this class from the world renowned IIT is excellent
Quantum Tunneling
Quantum tunneling allows particles to pass through energy barriers that would be classically insurmountable. Think of it as a particle somehow appearing on the other side of a high, impermeable wall without having the classical energy to climb over it—simply bypassing conventional physical constraints. Tunneling underlies many biochemical reactions, including enzyme catalysis, indicating that biology may inherently exploit quantum effects.
One of my favorite videos on Quantum Tunneling
Microtubules as Quantum Computers: How Might They Work?
Tubulin Proteins and Quantum Dipoles
Central to Orch OR is the idea that the protein subunits of microtubules—α- and β-tubulin—could behave as quantum dipoles, possessing intrinsic electrical polarity. Due to their molecular structure, tubulin proteins contain regions that create permanent or transient dipoles—molecules with separated positive and negative charges that oscillate rhythmically. These dipolar oscillations, when synchronized, could theoretically form quantum coherent states.
This coherence could, under certain conditions, extend across multiple microtubules and even interconnected neuronal networks. The result could be quantum coherence patterns, effectively allowing microtubules to function analogously to quantum computational systems.
Quantum Computations via Resonance
Hameroff and Penrose propose that microtubules could support quantum coherence through resonant vibrational modes. Just as a tuning fork maintains resonance at specific frequencies, microtubules—because of their periodic and symmetrical lattice structure—could sustain coherent vibrational resonances, possibly in the terahertz, gigahertz, and megahertz ranges. These resonances might effectively shield quantum states from rapid decoherence (the collapse of coherent quantum states into classical ones), a crucial requirement for sustaining quantum computations at biological temperatures.
Experimental Evidence and Critical Perspectives
Supportive Evidence: Bandyopadhyay’s Quantum Resonance Experiments
Anirban Bandyopadhyay, a leading experimental physicist in quantum biology, provided groundbreaking empirical support for Orch OR through meticulous experiments. Using advanced scanning tunneling microscopy (STM) and dielectric spectroscopy, Bandyopadhyay’s team demonstrated that microtubules exhibit remarkable quantum-like resonances at biologically relevant temperatures. They observed clear electrical conductivity and coherent oscillations in tubulin dimers, microtubules, and even networks of interconnected microtubules—finding resonances at distinct frequencies predicted by theoretical models. These findings provided the first substantial experimental indication that quantum coherence could indeed manifest robustly within biological structures at normal physiological conditions.
Criticism and Counterarguments
Despite intriguing evidence, Orch OR faces substantial criticism from some physicists, notably MIT’s Max Tegmark. Tegmark and others argue that quantum coherence at room temperature is extremely short-lived (on the order of picoseconds), rendering sustained quantum computations biologically impossible. They propose that rapid decoherence would instantaneously collapse quantum states into classical ones, eliminating any meaningful quantum computational capabilities at physiological temperatures.
In response, Hameroff and Bandyopadhyay emphasize the role of resonant vibrational modes as a potential solution. They argue that microtubule vibrations create an environment of quantum error-correction, significantly prolonging coherence times and effectively mitigating decoherence effects. They propose that these resonant modes essentially shield quantum states from environmental disturbances, allowing quantum computations to persist at biologically relevant timescales.
Implications for Longevity Medicine and Neuroscience
The implications of quantum biology—and Orch OR in particular—for longevity medicine and neuroscience are profound. If consciousness and higher cognitive processes depend in part on quantum coherence within microtubules, this suggests potential novel therapeutic avenues:
Neurodegenerative Diseases: If quantum coherence disruptions underlie cognitive decline, therapies restoring or enhancing quantum coherence in microtubules may offer transformative approaches to Alzheimer's, Parkinson's, and other age-related neurodegenerative diseases.
Enhanced Cognitive Longevity: Understanding microtubular quantum mechanics may guide innovative strategies to preserve cognitive function with aging, potentially through external stimulation or pharmacological modulation of microtubule resonances.
Anesthetic and Consciousness Studies: Exploring quantum effects in microtubules provides a compelling biological basis for anesthetic mechanisms, as anesthetics might disrupt microtubule quantum coherence, causing loss of consciousness. This perspective offers a more unified biological framework for consciousness and anesthesia, invaluable to both clinical and theoretical neuroscience.
Quantum Biology—A Frontier Worth Exploring
Quantum biology, particularly the Orch OR theory of microtubular quantum consciousness, challenges traditional boundaries between physics and biology. By considering quantum coherence, entanglement, and tunneling within biological contexts, scientists and clinicians may unlock new understandings and treatments of aging, cognition, and consciousness itself.
Though controversies remain, the evidence increasingly supports a role for quantum mechanics in biological systems. For longevity physicians, quantum biology represents a transformative frontier, opening entirely new therapeutic possibilities aimed at maintaining cognitive function, delaying neurodegeneration, and fundamentally deepening our comprehension of human biology.
24 minutes of Quantum Biology. Watch this one for sure!
Clinical Implications and Future Therapeutics—Microtubules in Longevity Medicine and Quantum Therapeutics
Introduction: Bridging Basic Science and Clinical Application
Understanding microtubules at a molecular, cellular, and even quantum mechanical level opens the door to revolutionary therapeutic interventions in longevity medicine. Historically considered as passive cellular scaffolds, microtubules have emerged as active participants in aging-related diseases, particularly neurodegenerative disorders. Now we will explore the current state of microtubule-targeted therapies, the speculative but compelling domain of quantum therapeutics, and the emerging use of transcranial ultrasound technology—particularly in Europe, and our own office—as a potential means to enhance cognitive longevity via microtubule coherence.
Microtubules as Therapeutic Targets in Longevity Medicine
The clinical significance of microtubules is anchored in their critical roles in maintaining cellular health—especially neuronal and immune function—which deteriorate significantly with aging. By strategically targeting microtubules, clinicians and researchers can potentially alter fundamental processes involved in cellular senescence, neurodegeneration, immunosenescence, and chronic inflammation ("inflammaging"). Current therapeutic developments focus on pharmacologically stabilizing microtubules, thereby enhancing their resilience against aging-related disruptions.
Current and Emerging Microtubule Therapeutics
1. Epothilones (Epothilone D and analogs)
Epothilones, mentioned earlier are potent microtubule-stabilizing agents originally developed as chemotherapy drugs (e.g., Ixabepilone). Unlike traditional taxanes, epothilones cross the blood-brain barrier effectively, making them particularly suitable for neurological applications. Epothilone D (BMS-241027) is currently in clinical trials for Alzheimer's disease, demonstrating an ability to restore microtubule integrity, improve axonal transport, and decrease tau aggregation. These drugs represent a paradigm shift in Alzheimer's treatment, moving beyond symptom relief to directly protecting neuronal structures.
2. HDAC6 Inhibitors
Histone Deacetylase 6 (HDAC6) plays a crucial regulatory role in microtubule acetylation, a post-translational modification enhancing microtubule stability. HDAC6 inhibitors, such as Tubastatin A, promote microtubule acetylation, thereby stabilizing neuronal microtubules and restoring impaired axonal transport observed in neurodegenerative conditions like amyotrophic lateral sclerosis (ALS). These compounds have shown promising results in preclinical models, significantly slowing neuronal degeneration, preserving neuromuscular function, and extending lifespan.
3. Noscapine Derivatives
Originally identified as a cough suppressant derived from opium poppies, noscapine uniquely stabilizes microtubules without significantly disrupting their dynamic behavior, a critical advantage for neuronal applications. Modified noscapine derivatives demonstrate potent neuroprotective effects in animal models of tauopathies and Parkinsonian syndromes. By protecting against tau hyperphosphorylation and maintaining microtubule integrity, these agents represent innovative therapeutic possibilities across multiple neurodegenerative diseases.
Quantum Therapeutics: Speculative Yet Intriguing Frontier
The recognition that quantum mechanical phenomena may influence biological systems at physiological conditions has opened novel therapeutic possibilities. The Orch OR theory, proposed by Sir Roger Penrose and Dr. Stuart Hameroff, posits that neuronal microtubules sustain quantum coherence, potentially underpinning cognitive functions and consciousness. If microtubules indeed perform quantum computations, therapeutic strategies aimed at enhancing or stabilizing these quantum states might profoundly improve cognitive longevity and mitigate age-related cognitive decline.
Therapeutic Potential of Enhancing Quantum Coherence:
Theoretically, maintaining or restoring quantum coherence in neuronal microtubules might prevent or reverse cognitive impairment seen in aging and neurodegenerative diseases. Pharmacological or biophysical approaches could be developed to optimize resonance conditions within neuronal microtubules, thus preserving quantum coherence. Such treatments might enhance memory, cognition, neuronal plasticity, and potentially even consciousness resilience.
Transcranial Ultrasound: Bridging Quantum Biology and Clinical Neuromodulation
As a brief Dr Murphy commentary (not medical advice, only my experience), I believe in this technology as I have seen great clinical success in myself as well as other biohackers. It is a powerful tool to stabilize and reassemble my thoughts. Many times in medicine we stumble upon scientifically sensible treatments that don’t pan out. But sometimes it is the side effects that trigger a need for further investigation and development. I have been performing transcranial doppler ultrasound for a decade now and what I couldn’t get over was the sense of peace patients felt after this study. They felt refreshed on cognitively improved as a side effect. We had no clue why at first. Then I learned about Quantum Biology and began to understand why this tool was immensely useful. Not just to look at the velocity of carotid siphons, but also to align our microtubules to enhance cognition and perhaps consciousness.
European Clinical Landscape—The EMA and Transcranial Ultrasound for Dementia
Europe, through the European Medicines Agency (EMA), has emerged as a leader in adopting innovative neuromodulation technologies, notably transcranial ultrasound (TUS), specifically transcranial pulse stimulation (TPS), to treat dementia. Unlike pharmaceuticals, TUS employs precisely targeted ultrasound waves non-invasively directed through the skull to stimulate deep brain structures. The EMA-approved NEUROLITH® TPS system (Storz Medical) is now clinically available in European centers for Alzheimer's disease, showing promising cognitive improvements and neuroprotective outcomes in multiple trials and clinical experiences.
How Does Transcranial Ultrasound Enhance Cognitive Function?
Transcranial ultrasound delivers mechanical waves that gently stimulate neurons and glial cells, enhance neurovascular coupling, and modulate neuroplasticity. Preclinical and clinical studies suggest it improves synaptic function, promotes the clearance of toxic proteins (e.g., amyloid-beta, pathological tau), reduces neuroinflammation, and stimulates neurogenesis—effects collectively beneficial for cognitive longevity.
Speculative Quantum Mechanism: Could Ultrasound Promote Microtubule Quantum Coherence?
Integrating insights from quantum biology with the clinical application of TUS leads to a provocative hypothesis: Could transcranial ultrasound stimulation, through resonance mechanisms, enhance microtubule quantum coherence? We have seen some clinical evidence of this in our day to day work with ultrasound.
Hypothetical Mechanism:
Microtubules exhibit intrinsic resonant frequencies due to their lattice-like structure and electrical dipoles.
Ultrasound, particularly at frequencies employed clinically (~0.5–3 MHz), might resonate with microtubular structures, potentially enhancing dipolar oscillations and quantum coherence states.
Sustained coherence could facilitate quantum computations within neuronal networks, possibly underpinning improvements in cognition, memory, and even conscious experience.
Clinical Implications of Quantum TUS: If experimental evidence confirms this mechanism, clinicians could use TUS not merely as symptomatic therapy but as a novel modality aimed at enhancing neuronal quantum coherence directly. This approach might revolutionize dementia treatments, potentially becoming a routine cognitive "maintenance" therapy, akin to annual cognitive check-ups or "brain fitness" interventions.
Leading Researchers and Institutions to Watch
Physicians interested in longevity medicine and quantum biology should closely monitor developments from pioneering investigators and institutions:
Eva Nogales (UC Berkeley): Structural biology expert elucidating the atomic structure and dynamics of microtubules and tau proteins, crucial for drug development.
Stuart Hameroff (University of Arizona) & Roger Penrose (University of Oxford): Leading theorists behind the Orch OR hypothesis, continuously refining the quantum consciousness model and implications for medical applications.
Virginia Lee (University of Pennsylvania): Renowned neuropathologist whose work illuminates the molecular mechanisms of tau aggregation and microtubule dysfunction in Alzheimer’s and related tauopathies.
Anirban Bandyopadhyay (National Institute for Materials Science, Japan): Experimental physicist whose research provides empirical support for microtubule quantum coherence at physiologically relevant conditions, bridging physics and biology.
Roland Beisteiner (Medical University of Vienna): Neurologist instrumental in clinical studies of transcranial ultrasound therapy for Alzheimer's, demonstrating safety and cognitive benefits.
A Vision for the Future of Longevity Medicine
Microtubules, once seen merely as structural supports, now occupy the forefront of clinical and scientific exploration in longevity medicine. Therapies stabilizing microtubules already show significant promise in aging-related diseases, and speculative quantum coherence mechanisms open entirely novel therapeutic frontiers. Coupling quantum biology insights with advanced clinical technologies such as transcranial ultrasound could create unprecedented opportunities for cognitive health and longevity.
Longevity physicians today stand at the threshold of this revolutionary integration—positioned uniquely to harness these scientific breakthroughs, fundamentally redefining strategies to preserve brain health, enhance resilience, and profoundly extend healthy cognitive lifespan.
Now would you like to seen a clinical example with protocols of how to use energy medicine to target microtubules?
Yes?
Keep reading!
Keep reading with a 7-day free trial
Subscribe to Longevity Insider with Dr. Murphy to keep reading this post and get 7 days of free access to the full post archives.